Autonomous Safety Intelligence
Beyond automation. True cognitive processing.
Experience the next evolution of pharmacovigilance. Our AI agents don't just process data; they understand it, ensuring speed, accuracy, and compliance without the fatigue.
How it works
Save time with AI collection and triage - start reviewing all your sources and finish by authorizing E2B-ready reports.
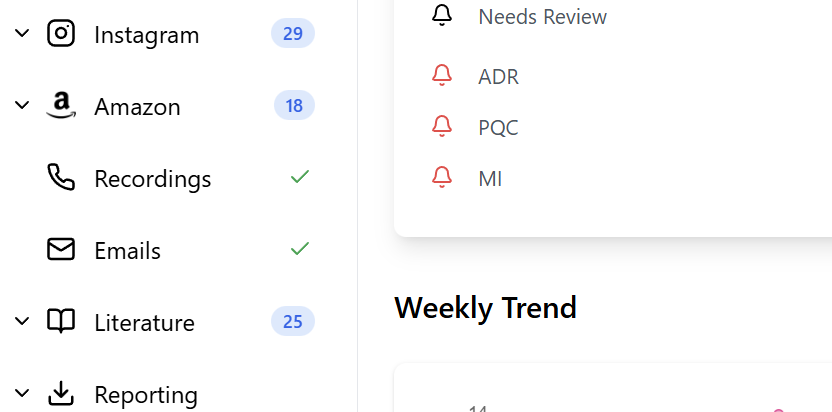
Check all your sources
Start the day by checking new patients reporting in all your channels - spontaneous reports, literature, call centers, emails, e-commerce reviews, social media, and more.
Let AI triage first
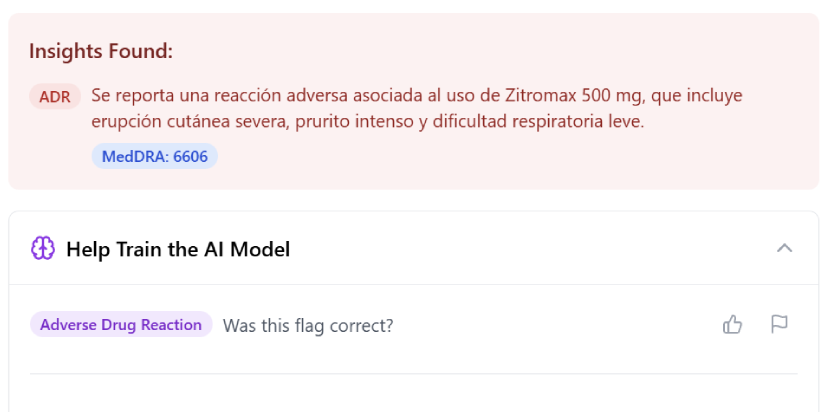
Our AI continuously scans and detects potential adverse events, safety signals, and safety information. It flags new findings, updates old entries, and lets you configure custom triggers to focus on what matters.
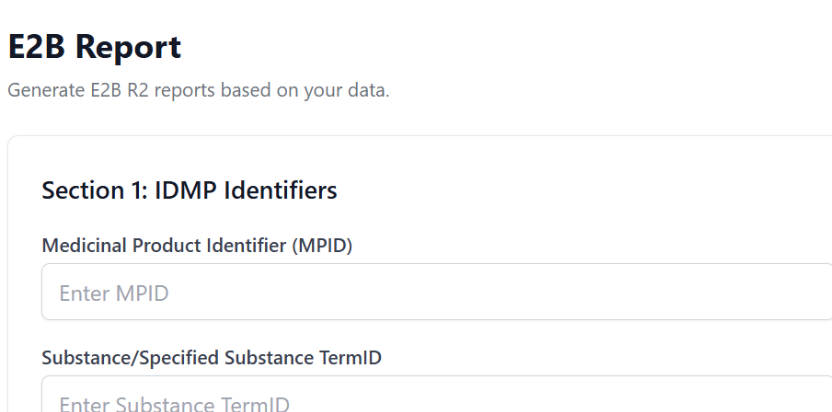
Transform findings into E2B
Once you validate a case, click -Convert to case-. AI will assist with translation and redaction - helping generate E2B reports in minutes.
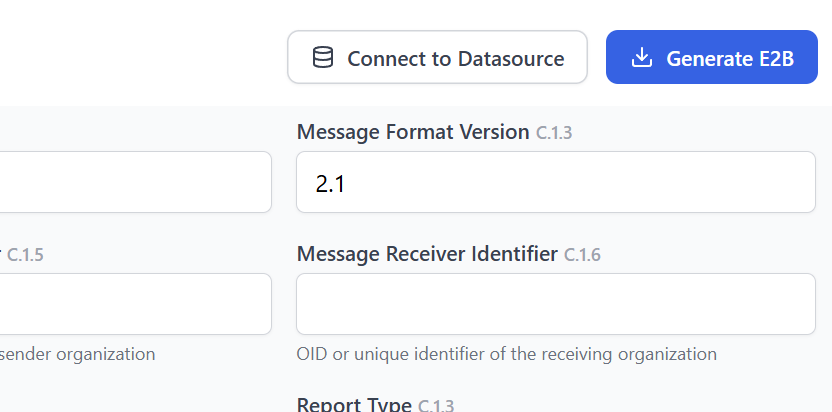
Export to database and follow-up
Seamlessly export findings to your safety systems, whether it is Argus, ARISg, VigiFlow, or others. You can also export E2B XML files directly for submission or import.
Accelerated
Slash intake times. Our agents instantly extract, triage, and route adverse events, separating routine cases from critical signals in milliseconds.
Cognitive
Context-aware analysis. Moving beyond simple OCR, we utilize advanced LLMs to interpret nuance, medical context, and unstructured narratives with human-like precision.
Elevated
Operational excellence. Free your experts from data entry to focus on safety science. Build a resilient, scalable safety organization for the future.
GVP-Compliant by Design
Engineered from the ground up for GVP and GxP adherence. We don't just support compliance; we enforce it.
AI takes care of step one, so you can focus on reviewing safety insights.
Built on Six Key Principles
Transforming pharmacovigilance workflows with unparalleled speed and compliance.
Zero-Touch Automation
Automate E2B(R3) generation and submission with complete auditability.
Cognitive Extraction
Parse complex, unstructured medical narratives with human-level understanding.
Adaptive Intelligence
Dynamic workflows that evolve with your specific safety processes and needs.
Sovereign Security
Your data stays yours with full encryption and zero-retention architecture.
Regulatory Guardrails
Built-in compliance for GVP, HIPAA, and SOC 2 standards out of the box.
Infinite Scalability
Handle any volume of adverse events with a multi-agent system that never sleeps.