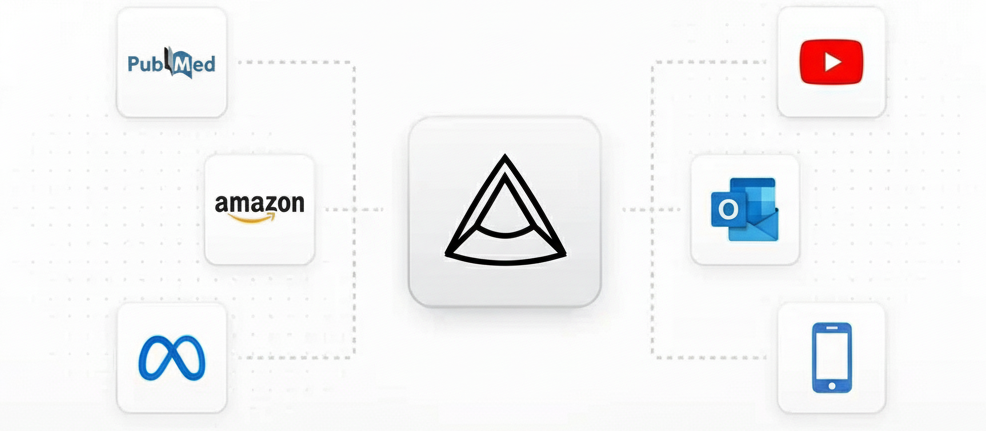
Plug-and-play integration with your safety stack
Complete intake coverage
From the Must-Haves to the Future-Proofing. We cover every channel where safety data exists.
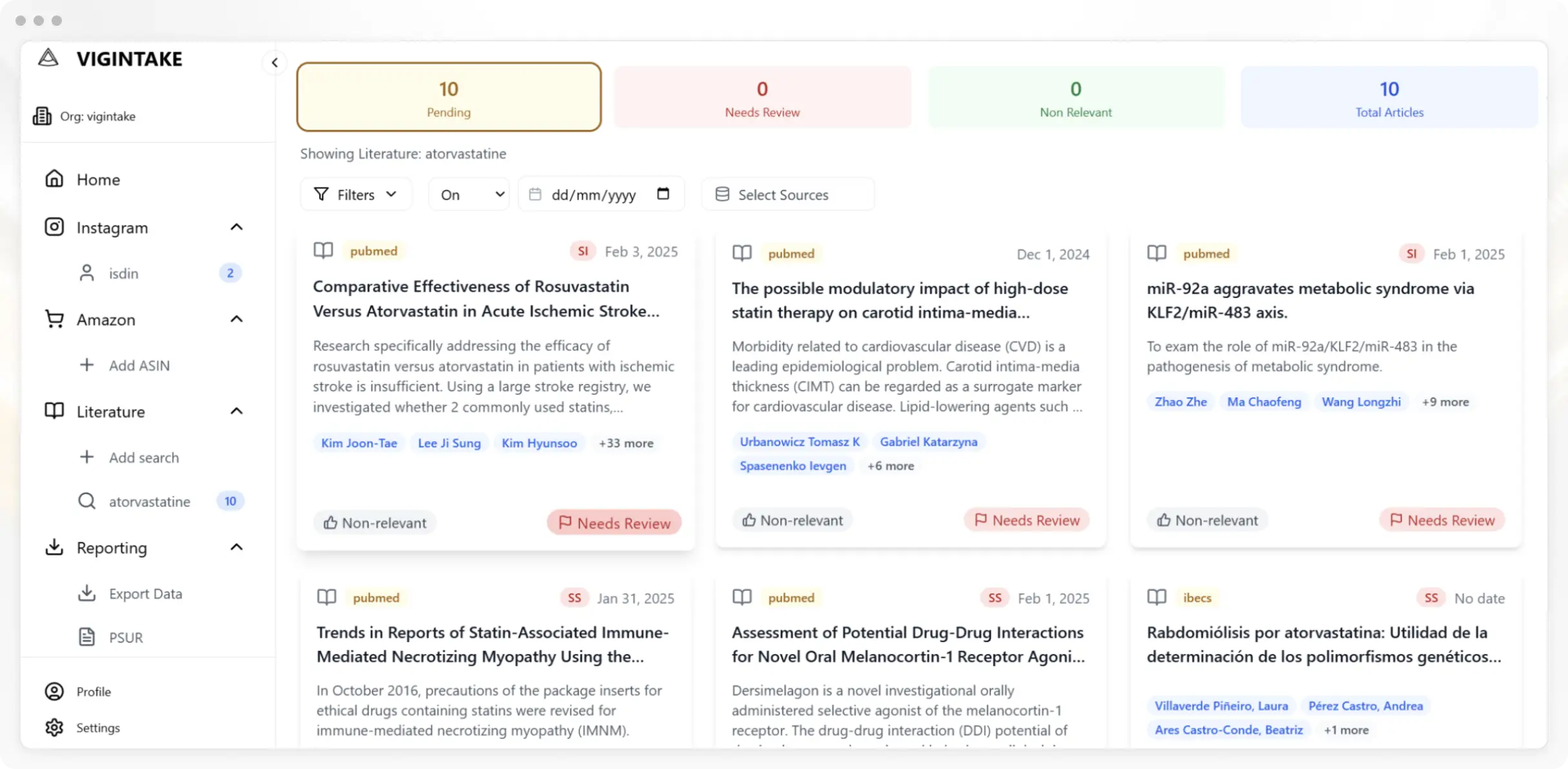
Take Control of Your PV Data
Vigintake empowers modern pharmacovigilance teams with the controls to reduce costs, stay in compliance, and rapidly onboard new channels with full traceability.
Reduce Review Volume
Reduce review volume by 90% before it arrives at your destination for analysis. Vigintake uses Human-In-The-Loop (HITL) to ensure accuracy and compliance.
Digital Records in One Place
Say goodbye to multiple logins, screenshots and spreadsheets. All in one place.
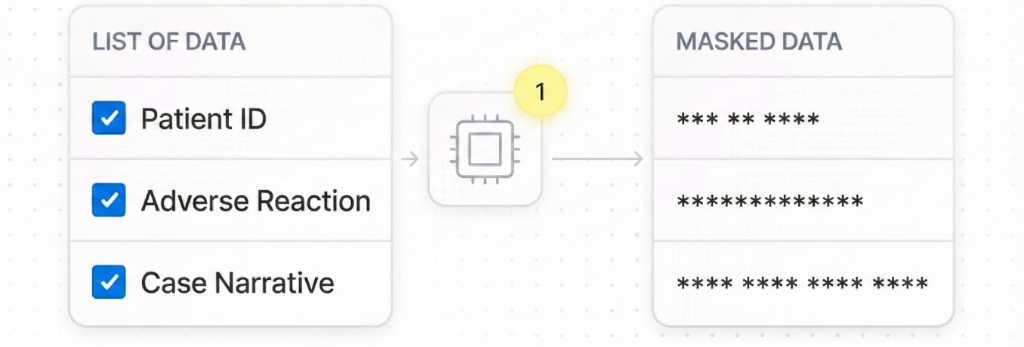
Ensure Data Privacy
Encrypt sensitive data before it leaves your servers, ensuring GDPR and GVP compliance.
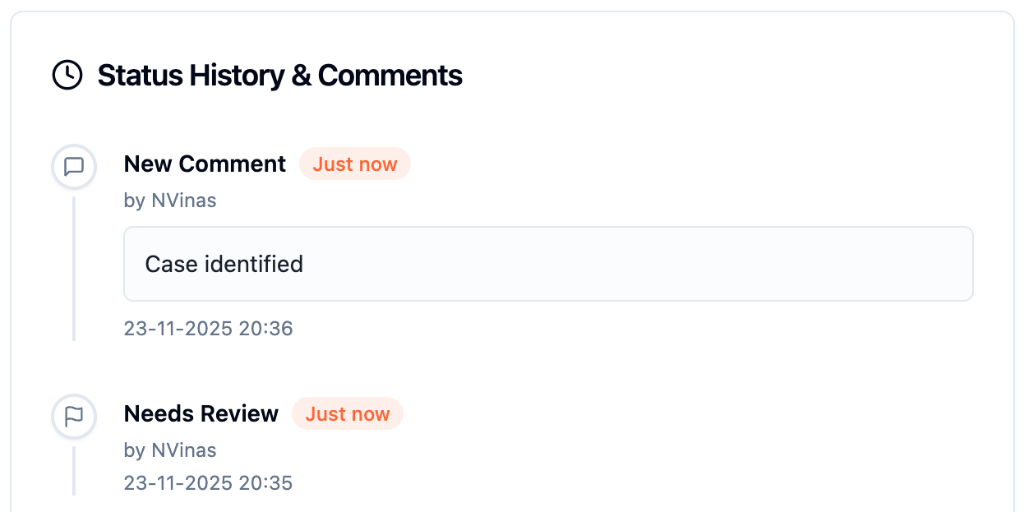
Seamless Platform Traceability
Vigintake provides a seamless platform traceability for audit inspections.
Vigintake is designed for
Scale without limits
Handle 10x the case volume with zero compromise on quality. Deliver faster, more accurate results that make your clients wonder how you do it.
Outperform the competition
Stop drowning in journals. Our AI finds what manual screening misses, giving you complete coverage and happier pharma clients.
Take back control
Bring PV in-house. Cut contractor dependency, eliminate compliance gaps, and own your safety intelligence from end to end.
Validated. Secure. Audit-Ready
At Vigintake, security, compliance, privacy, and transparency are core to our platform. We protect customer data through industry leading security controls, independent audits, and strict adherence to global compliance standards.
FDA 21 CFR Part 11 (Electronic Records and Electronic Signatures)
EU GAMP Annex 11 (Computerised Systems)
ICH E6(R2) Good Clinical Practice
ISO 14155 (Clinical investigation of medical devices)
GDPR (General Data Protection Regulation)